Tap to see IMPORTANT SAFETY INFORMATION AND INDICATION
Important Safety Information for PROMACTA® (eltrombopag)
WARNING: RISK FOR HEPATIC DECOMPENSATION IN PATIENTS WITH CHRONIC HEPATITIS C
In patients with chronic hepatitis C, PROMACTA in combination with interferon and ribavirin may increase the risk of hepatic decompensation.
RISK OF HEPATOTOXICITY
PROMACTA may increase the risk of severe and potentially life-threatening hepatotoxicity. Monitor hepatic function and discontinue dosing as recommended.
WARNING: RISK FOR HEPATIC DECOMPENSATION IN PATIENTS WITH CHRONIC HEPATITIS C
In patients with chronic hepatitis C, PROMACTA in combination with interferon and ribavirin may increase the risk of hepatic decompensation.
RISK OF HEPATOTOXICITY
PROMACTA may increase the risk of severe and potentially life-threatening hepatotoxicity. Monitor hepatic function and discontinue dosing as recommended.
Additional Information Regarding Hepatic Decompensation in Patients With Chronic Hepatitis C
- In 2 controlled clinical trials in patients with chronic hepatitis C and thrombocytopenia, ascites and encephalopathy occurred more frequently with PROMACTA plus antivirals (7%) than antivirals alone (4%)
- Patients with low albumin levels (<3.5 g/dL) or model for end-stage liver disease score ≥10 at baseline had a greater risk for hepatic decompensation with PROMACTA plus antivirals
- Discontinue PROMACTA if antiviral therapy is discontinued
Hepatotoxicity
PROMACTA may increase the risk of severe and potentially life-threatening hepatotoxicity.
Treatment of ITP, chronic hepatitis C, and refractory severe aplastic anemia
- Measure serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin prior to initiation of PROMACTA, every 2 weeks during the dose-adjustment phase, and monthly following establishment of a stable dose
- PROMACTA inhibits UGT1A1 and OATP1B1, which may lead to indirect hyperbilirubinemia. If bilirubin is elevated, perform fractionation
- Evaluate abnormal serum liver tests with repeat testing within 3 to 5 days. If the abnormalities are confirmed, monitor serum liver tests weekly until resolved or stabilized
- Discontinue PROMACTA if ALT levels increase to ≥3 times the upper limit of normal in patients with normal liver function or ≥3 times baseline in patients with pretreatment elevations in transaminases and are progressively increasing; or persistent for ≥4 weeks; or accompanied by increased direct bilirubin; or accompanied by clinical symptoms of liver injury or evidence for hepatic decompensation
- If the potential benefit for reinitiating treatment with PROMACTA outweighs the risk for hepatotoxicity, then consider cautiously reintroducing PROMACTA and measure serum liver tests weekly during the dose-adjustment phase. Hepatotoxicity may reoccur if PROMACTA is reinitiated. If liver test abnormalities persist, worsen, or recur, then permanently discontinue PROMACTA
First-line treatment of severe aplastic anemia
- ALT, AST, and bilirubin should be measured prior to initiation of PROMACTA
- During treatment, increases in ALT levels should be managed based on recommendation in Dosing and Administration section for hepatic impairment
Thrombotic/Thromboembolic Complications
- Thrombotic/thromboembolic complications may result from increases in platelet counts with PROMACTA
- Reported thrombotic/thromboembolic complications included both venous and arterial events, and were observed at low and at normal platelet counts
- Portal vein thrombosis has been reported in patients with chronic liver disease receiving PROMACTA
- To minimize the risk for thrombotic/thromboembolic complications, do not use PROMACTA in an attempt to normalize platelet counts. Follow the dose-adjustment guidelines to achieve and maintain target platelet counts
Increased Risk of Death and Progression of Myelodysplastic Syndromes (MDS) to Acute Myeloid Leukemia (AML)
- In a clinical trial of patients with intermediate- to high-risk MDS and thrombocytopenia receiving PROMACTA, an increased number of progressions from MDS to AML and deaths have been observed compared to placebo
- PROMACTA is not indicated for the treatment of patients with MDS
Cataracts
- Development or worsening of cataracts with PROMACTA has been reported with a frequency of 5% to 11% in 6 clinical studies
- Perform a baseline ocular examination prior to initiating PROMACTA. Regularly monitor patients for signs and symptoms of cataracts while on PROMACTA
Laboratory Monitoring
Persistent or chronic ITP
- Monitor serum liver tests
- During therapy with PROMACTA, assess complete blood counts (CBCs) with differentials, including platelet counts, weekly until a stable platelet count has been achieved. Monitor platelet counts monthly thereafter
- Obtain CBCs with differentials, including platelet counts, weekly for at least 4 weeks following discontinuation of PROMACTA
- When switching between the oral suspension and tablet, assess platelet counts weekly for 2 weeks, then follow standard monthly monitoring
Chronic hepatitis C-associated thrombocytopenia
- Monitor serum liver tests
- Monitor CBCs with differentials, including platelet counts, every week prior to starting antiviral therapy. Obtain CBCs with differentials, including platelet counts, weekly during antiviral therapy until a stable platelet count is achieved, then monitor platelet counts monthly thereafter
Severe aplastic anemia
- Monitor serum liver tests
- Monitor clinical hematology tests regularly throughout therapy with PROMACTA and modify the dosage regimen of PROMACTA based on platelet counts
- Hematologic response may take up to 16 weeks after starting PROMACTA. If no hematologic response has occurred after 16 weeks with PROMACTA, discontinue therapy
Drug/Drug and Drug/Food Interactions
- PROMACTA must be taken at least 2 hours before or 4 hours after any medications or products containing polyvalent cations such as antacids, calcium-rich foods, and mineral supplements
- Take PROMACTA without a meal or with a meal low in calcium (≤50 mg)
Adverse Reactions
Across all indications, the most common adverse reactions (≥20% in any indication) were anemia, nausea, pyrexia, ALT increased, cough, fatigue, headache, and diarrhea.
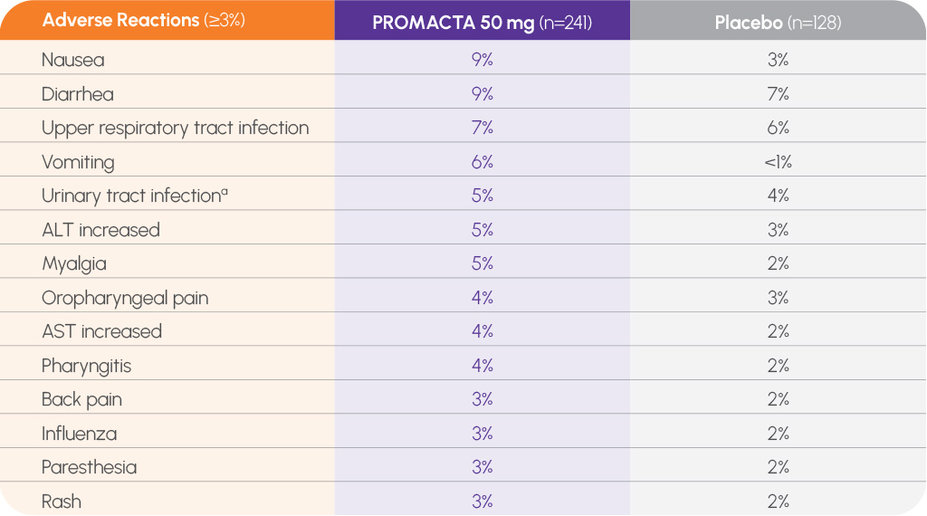
The most common adverse reactions in 3 placebo-controlled clinical trials in patients with persistent or chronic ITP (≥3% and greater than placebo) for PROMACTA were nausea (9%), diarrhea (9%), upper respiratory tract infection (7%), vomiting (6%), increased ALT (5%), myalgia (5%), urinary tract infection (5%), oropharyngeal pain (4%), increased AST (4%), pharyngitis (4%), back pain (3%), influenza (3%), paresthesia (3%), and rash (3%).
The most common adverse reactions in 2 placebo-controlled clinical trials in patients with persistent or chronic ITP 1 year and older (≥3% and greater than placebo) for PROMACTA were upper respiratory tract infection (17%), nasopharyngitis (12%), cough (9%), diarrhea (9%), pyrexia (9%), abdominal pain (8%), oropharyngeal pain (8%), toothache (6%), ALT increased (6%), rash (5%), AST increased (4%), and rhinorrhea (4%).
The most common adverse reactions in 2 randomized placebo-controlled clinical trials in thrombocytopenic patients with chronic hepatitis C (≥10% and greater than placebo) for PROMACTA were anemia (40%), pyrexia (30%), fatigue (28%), headache (21%), nausea (19%), diarrhea (19%), decreased appetite (18%), influenza-like illness (18%), asthenia (16%), insomnia (16%), cough (15%), pruritus (15%), chills (14%), myalgia (12%), alopecia (10%), and peripheral edema (10%).
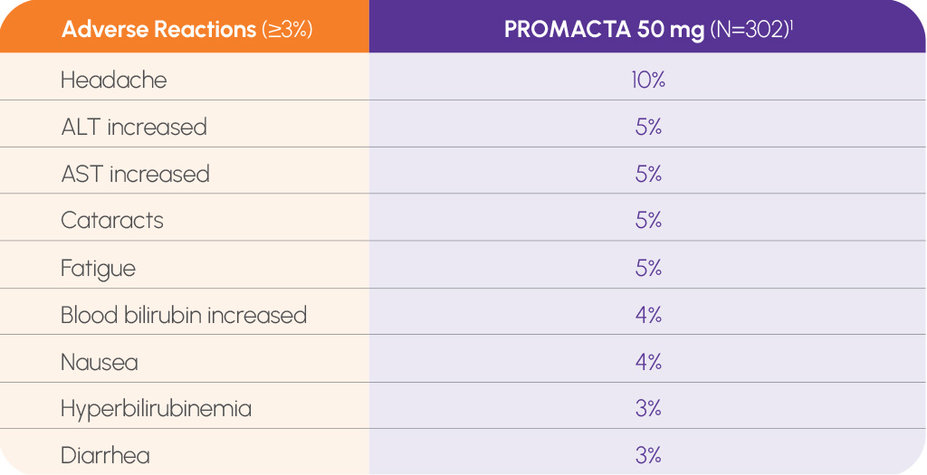
The most common adverse reactions (≥5%) in a single-arm trial of 92 patients 2 years and older with severe aplastic anemia (SAA) who had not received prior immunosuppressive therapy were increased ALT (29%) and AST (17%), blood bilirubin increased (17%), rash (8%), and skin discoloration including hyperpigmentation (5%). Upper respiratory infection, particularly in pediatric patients, was also reported. The most common adverse reactions (≥20%) in a single-arm, open-label trial in 43 patients with refractory SAA who received PROMACTA were nausea (33%), fatigue (28%), cough (23%), diarrhea (21%), and headache (21%). In this trial, patients had bone marrow aspirates evaluated for cytogenetic abnormalities. Eight patients had a new cytogenetic abnormality reported, including 5 patients who had complex changes in chromosome 7. If new cytogenetic abnormalities are observed, consider discontinuation of PROMACTA.
Please see full Prescribing Information, including Boxed WARNING, and Medication Guide.